Does Reverse Osmosis Remove Chloramine? In this post, we will explore the effectiveness of RO systems in removing chloramine, looking at the relevant research and potential implications.
We will also provide some practical tips on how to maximize the removal of chloramine through RO systems. With this information, readers will be better equipped to make an informed decision about their water treatment options.
Chloramine is a combination of ammonia and chlorine that is commonly used as a disinfectant in drinking water. While it is effective at killing bacteria and other pathogens, it can also cause health concerns if ingested in large amounts. Reverse osmosis (RO) is a common drinking water treatment process used to remove contaminants from water.
What is Chloriamine?
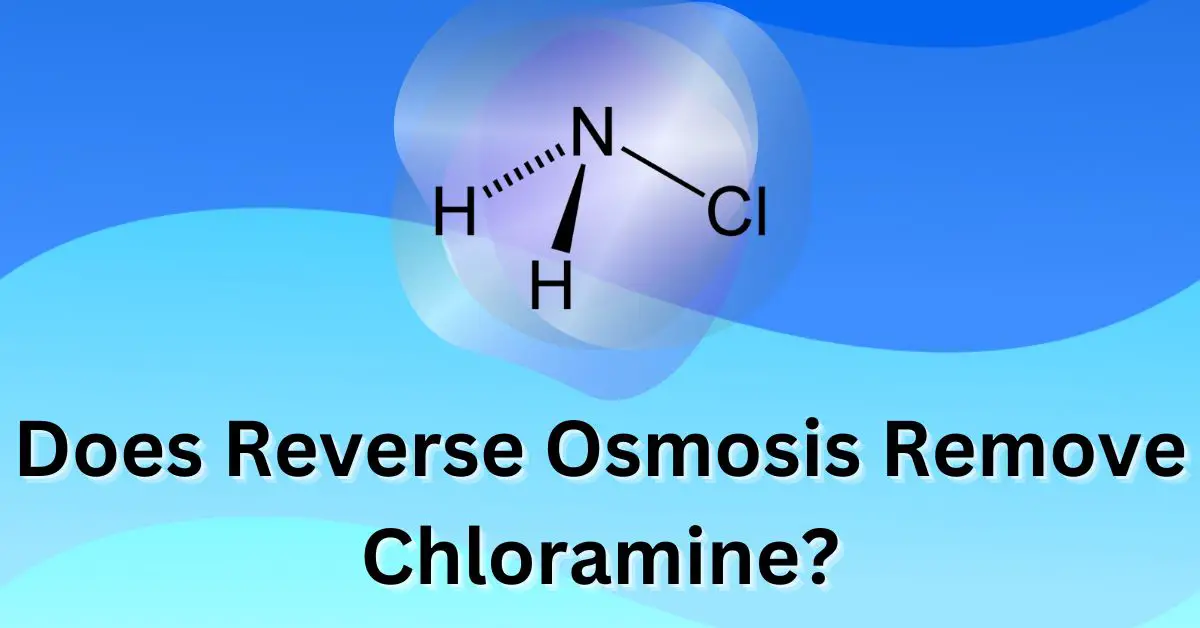
Chloramine is a chemical compound that is formed when chlorine is added to ammonia. It is a liquid or gas at room temperature, depending on the specific form, and has a pungent, bleach-like smell.
Chloramine is commonly used as a disinfectant in water treatment facilities to kill bacteria and other pathogens in drinking water. It is less effective at killing bacteria than chlorine, but it is more stable and has a longer shelf life, making it a popular choice for water disinfection.
Chloramine is also used in other applications, including as a disinfectant in swimming pools and spas, and as a cleaning agent in the food and beverage industry.
While chloramine is generally considered to be safe when used as a disinfectant in drinking water, it can have negative effects on human health if ingested in high amounts. It can cause digestive issues, such as nausea, vomiting, and diarrhea, and can irritate the skin, eyes, and respiratory system. Chronic exposure to high levels of chloramine may also increase the risk of cancer and other health problems.
Types of Chloramines Found in Water
Chloramines are chemical compounds that are formed when chlorine is added to ammonia. There are two main types of chloramines that can be found in water: monochloramine and dichloramine.
- Monochloramine: Monochloramine, also known as monochloramine, is formed when one molecule of chlorine is added to one molecule of ammonia. It is the most common form of chloramine used in water treatment and is generally considered to be the most effective at killing bacteria and other pathogens in drinking water.
- Dichloramine: Dichloramine, also known as dichloramine, is formed when two molecules of chlorine are added to one molecule of ammonia. It is less stable and less effective at killing bacteria than monochloramine, but it is more stable than chlorine and can have a longer shelf life.
Both monochloramine and dichloramine are commonly used as disinfectants in water treatment facilities to kill bacteria and other pathogens in drinking water.
They are generally considered to be safe when used at appropriate levels, but they can have negative effects on human health if ingested in high amounts. It is important to follow the guidelines for the safe use of chloramines in water treatment to ensure that the water is safe to drink
Effect of Chloramine in The Human Body
Chloramine is a chemical compound that is formed when chlorine is added to ammonia. It is commonly used as a disinfectant in water treatment facilities to kill bacteria and other pathogens in drinking water.
Exposure to chloramine can have negative effects on the human body, particularly if it occurs at high levels or over an extended period of time. When ingested, chloramine can cause digestive issues, such as nausea, vomiting, and diarrhea. It can also irritate the skin, eyes, and respiratory system, leading to symptoms such as redness, itching, and difficulty breathing.
Long-term exposure to chloramine or chloramine compounds may also increase the risk of cancer and other health problems. Some studies have suggested that chloramine exposure may increase the risk of bladder, kidney, and pancreas cancer, as well as asthma and other respiratory issues.
The effects of chloramine on the body depend on the level of exposure and the duration of exposure. Most people can tolerate low levels of chloramine in their drinking water without experiencing any negative effects.
How Does Reverse Osmosis Remove Chloramine From Water?
Reverse osmosis is a process that is used to remove a variety of contaminants from water, including chloramine. The process works by using a semi-permeable membrane to filter out contaminants and impurities from the water.
The membrane is made up of very small pores that only allow water molecules to pass through, while larger molecules, such as chloramine, are left behind. As the water passes through the membrane, the chloramine is removed from the water, leaving it pure and clean.
In conclusion, reverse osmosis is an effective way to remove chloramine from water. It also removes other contaminants, such as lead, arsenic, fluoride, and nitrate, which can all be health hazards.
Reverse osmosis is an effective and cost-effective solution for those who need to remove chloramine from their water. It is important to note, however, that the process requires regular maintenance and upkeep, which can be costly.