What is DM Plant?
DM plant full form is Demineralization plant.
The ion exchange process is used to remove mineral salts or dissolved ions from water, which is known as demineralization or deionization.
Demineralization can be used with most natural water sources to produce water of greater quality than traditional distillation process. Only dissolved solids are removed in DM Plant.
Water contains both positive and negative charged ions.
Positive charged ions like calcium, magnesium, sodium, potassium etc. called cations.
Negative charged ions like chloride, sulfate, carbonate, bicarbonate etc. called anions.
DM Plant Process:
First, Understand five useful abbreviation of DM plant which are frequently used.
SAC – Strong Acid Cation
WAC- Weak Acid Cation
DG or DGT – Degasser Tower
SBA – Strong Base Anion
WBA- Weak Base Anion
The complete DM plant process divided in four parts called cation exchange, degassification, anion exchange and mixed bed polishing process.
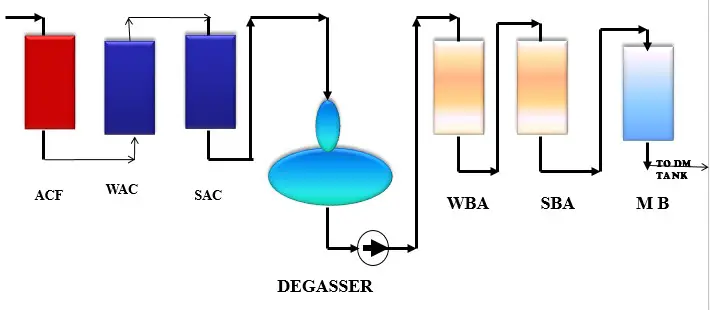
1. Cation Exchange Process:
Cation resin has a negative functional group therefore it attracts positive charged ions called cations.
Cation exchanger vessel is constructed with steel inside rubber lined. It has 50 to 75% void space to allow free expansion of resin during back wash.
Cation Exchange process contains two types of exchanger namely SAC & WAC.
Weak Acid Cation (WAC) Exchanger:
When raw water contain high amount of temporary hardness then Weak Acid Cation (WAC) is followed with Strong Acid Cation (SAC)
This affinity series is equal to that of a SAC- exchanger, except for the hydrogen ion, which is standing in front. This means that this type of exchanger has the strongest affinity for the hydrogen-ion.
H+ > Fe+3 > Al+3 > Ca+2 > Mg+2 > K+ > Na+
Weak acid cation-exchangers can remove cations associated with bi-carbonate alkalinity only. The bi carbonates are converted into carbonic acid. They do not function well at pH levels below 5.0.
It performs better with high pH water, with lower pH water its performance decreases and when pH falls below 4, actually regeneration takes place.
Strong Acid Cation (SAC) Exchanger :
The function of the strong acid cation exchanger is to remove cations from the water.
The CATION (positive charged ions) such as calcium, magnesium & sodium are replaced by HYDROGEN ion (H+).
Affinity of ions in SAC as per given below:
Fe+3 > Al+3 > Ca+2 > Mg+2 > K+ > Na+ > H+
This means that Fe+3 ions combine much more strongly with the resin than the Na+ ions.
In practice the Fe+3 ions will supersede ions of aluminum, calcium, magnesium, etc. from the exchanger and Ca+2 ions will supersede the ions of magnesium, potassium, sodium and hydrogen.
The cation exchanger in the hydrogen cycle means that almost all mobile cations within the
exchanger are hydrogen ions.
Strong acid cation exchangers are able to convert neutral salts into their corresponding acids like HCl, H2SO4 etc. Cation exchanger generates acidic water.
SAC function well at all pH ranges.
2. Degassifier
The effluent is acid after the cation exchanger, and all of the bicarbonate in the water is converted to CO2.
This CO2 may be eliminated for a very low cost in a Degasser. A Degasser has a low initial investment and ongoing operating costs. Otherwise, CO2 will be extracted via an anion exchanger. As a result, the Degasser lessens the load on the anion exchanger.
There are different types of degasser. The unit essentially consists of a tower mounted on a tank to hold 1-2 minutes supply of degassified (decarbonated) water.
Degasser internal packing of wooden slant trays or ceramic raschig rings or ceramic / plastic ball rings etc. over which water trickles down from a spray pipe (corrosion resistant) at the top. Filtered air is blown in at the bottom and rises counter current to the downward water.
The spray and packing divide the water into droplets or thin films, exposing new surfaces to the gas phase. Packing materials also provide agitation am thus allows the dissolved gases to leave the water readily.
Packing also provides adequate detention time for near approach to equilibrium solubility of the gas in water. The height required for packing material ~ when the amount of influent CO2 is higher, when the amount of effluent CO2 derived is lower and when the water temperature is lower.
In general, economics favor use of a forced-draft degasifier when the carbon dioxide content (including bicarbonate alkalinity) is greater than 50 ppm and the service flow rate is greater than 50 gal./min.
For bicarbonate alkalinity plus CO2 loads of less than 50 ppm, and/or for flow rates less than 50 gpm, the economics do not generally favor the use of a degasifier.
Because degasifiers reduce the overall load on the anion resin, they result in a decreased quantity
of anion resin. The reduction in CO2 load increases the percentage of silica and organics,
increasing the risk of silica fouling and of organic fouling.
Normally, degassifier is designed to reduce CO2 level to less than 5 ppm.
3. Anion Exchange Process:
The function of the anion exchange process is to remove anions from the water. Constructional features of anion exchanger are similar to cation exchanger unit.
Anion resin has a positive function group attached therefore it attracts negative charged ions called anions.
Weak Base Anion (WBA) Exchanger:
Affinity of ions in WBA as per given below:
OH– > SO4-2 > NO3– > Cl–
Weak-base anion-exchangers are essentially mineral acid neutralisers. The degassed water from degassed water storage tank is coming to the weak base anion unit. The anions like chlorides and sulphates are removed in this unit are removed in this unit by ion exchange process.
These resins will not remove weak acids such as silicic acid and carbonic acid.
They work most efficiently at pH ranges below 4.0.
Strong Base Anion (SBA) Exchanger:
Affinity of ions in SBA as per given below:
S04-2 > NO3– > Cl– > HC03– > HSiO3– > OH–
The weak base anion unit’s outlet is connected to the Strong base anion unit. The anions such as silicates, bicarbonates, and residues of anions leaking from WBA are eliminated in this unit by an ion exchange method.
Strong-base anion-exchangers in the hydroxide cycle are able to convert neutral salts into their
corresponding bases. The reaction can be represented as follows:
The anion exchanger in the hydroxide cycle means that almost all mobile anions within the
exchanger are hydroxide ions.
During operation, effluent coming out from anion out let contain some silica as leakage. At the end point of run silica leakage starts increasing and at a preset value the Unit is taken out of service for regeneration.
Strong-base anion-exchangers can remove anions of both strong and weak acids.
They can operate over the entire pH range.
4. Mixed Bed System:
In a mixed bed system, strong acid cation and strong base anion resins are intermixed. This basically transforms the mixed bed tank into a tank with thousands of dual bed units.
The cation and anion exchange process occurs repeatedly inside the mixed bed. Because of the large number of cation/anion exchanges that occur, sodium leakage is addressed.
You may generate the finest quality deionized water possible by using a mixed bed.
Quality of Effluent Water:
The quality of effluent water of SAC, Degasifier, SBA & MB will have the following property.
SAC Outlet Water Parameter
| pH at 25 ℃ | 2.8 to 3.5 |
| Free Mineral Acidity(FMA) | Should be Equal to Equivalent Mineral Acidity(EMA) |
| Hardness ppm as CaCO3 | NIL |
| Sodium ppm as Na+ | <0.005 |
Degasifier Outlet Water Parameter
| Residual free CO2 ppm | <5.0 |
SBA Outlet Water Parameter
| pH at 25 ℃ | 7.5 to 9.0 |
| Total silica SiO2 ppm | <0.05 |
| Conductivity(µs/cm) | < 2.0 preferably < 1.0 |
| Chloride ( ppm as Cl ) | NIL |
MB Outlet Water Parameter
| pH at 25 ℃ | 6.8 to 7.2 |
| Total silica SiO2 ppm | <0.02 |
| Conductivity(µs/cm) | < 0.2 |
| Sodium ppm as Na+ | <0.003 |
FAQs: DM Plant Process
1. What is the role of degasser in DM plant?
Degasser or Degassifier reduce the overall load on the anion exchange resin. It can reduce the CO2 level up to less than 5 ppm.
2. What is the full form of DM plant ?
Demineralization plant is the full form of dm plant.
What is OBR in DM plant?
OBR means Output Between Regeneration. OBR indicates the duration between two regeneration.
